STENTiT Enrolls First Patient in Clinical Trial Evaluating a Novel Stent with Regenerative Properties to Support Limb Preservation
www.stentit.com
STENTiT Enrolls First Patient in Clinical Trial
Evaluating a Novel Stent with Regenerative Properties to Support Limb Preservation
EINDHOVEN, The Netherlands – June 26, 2025 – STENTiT, a pioneering medical device company, developing a novel class of stents with regenerative properties, today announced the successful implantation of its Resorbable Fibrillated Scaffold (RFS). As part of the VITAL-IT 1 study, patients with chronic limb-threatening ischemia (CLTI) below-the-knee, have successfully been treated using the RFS implant. In this clinical procedure, revascularization was achieved using an endovascular approach to restore the blood flow to the foot
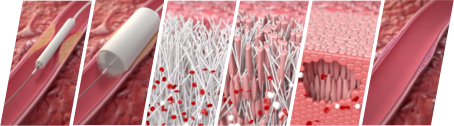
STENTiT’s RFS device is a bioresorbable stent built from microfibers, providing structural support to instantly open, and facilitate the reconstruction of the artery. Due to the porous design of the implant, patient’s own cells infiltrate into the mesh, triggering the formation of new vascular tissue. While the artery is being reconstructed from the inside-out, the synthetic implant gradually resorbs and ultimately disappears over time.
“Our goal is to bring the next generation of stents with regenerative properties, to guide the body rebuilding functional arteries and improve long-term clinical outcomes,” said Bart Sanders, CEO of STENTiT. “We are extremely proud reaching this first clinical milestone to show the potential of our technology in providing a durable solution for advanced peripheral artery disease.”
VITAL-IT 1 (NCT07006467) is a prospective, non-randomized feasibility study, designed to evaluate the STENTiT’s RFS device in up to 10 patients with below-the-knee CLTI. This single-center study is being conducted at the Medical University of Graz, Austria. All study patients will be monitored for 24 months.
“This first-in-human clinical study will provide an important indication on the translational potential of this new technology in CLTI patients,” said Prof. Marianne Brodmann, MD, Head of Division of Angiology at the Medical University of Graz. “This device combines key attributes of temporary structural support with regenerative properties, which could minimize the need for reinterventions.”
— ENDS —
About Chronic Limb Threatening Ischemia (CLTI) – CLTI is the end stage of peripheral artery disease (PAD) characterized by constant rest pain, non-healing ulcers, or gangrene caused by critically reduced blood flow to the foot. The condition affects an estimated 3.5 million people in Europe and the United States and leads to more than 250 000 amputations per year. Despite advances in the field, long-term patency in below-the-knee vessels remains poor, leaving many patients with limited durable treatment options.
About the Resorbable Fibrillated Scaffold (RFS) – The RFS is a fully bioresorbable microfiber stent, designed to restore blood flow to the foot in patients with chronic limb-threatening ischemia below-the-knee. The porous structure of the stent facilitates the infiltration of patients own blood-cells, to trigger a natural healing response facilitating the growth of new functional vascular tissue. While the artery is being rebuild from the inside-out, the implant gradually dissolves, leaving no foreign material behind. The RFS is currently undergoing clinical testing in the VITAL-IT 1 first-in-human study.
About STENTiT – STENTiT is a pioneering medical device company located at the High Tech Campus in Eindhoven, The Netherlands. Founded in 2017 as a spin-off from the Eindhoven University of Technology, STENTIT is developing a new class of stents with regenerative properties to set the new standard in cardiovascular treatment. The company is supported by the European Innovation Council’s EIC Accelerator program and has been selected for the 2025 MedTech Innovator cohort.
For more info please visit www.stentit.com, or find us on Linkedin
Technology Animation: Watch the video
NOTE TO EDITORS
FOR CONTACT
STENTiT | Eindhoven, The Netherlands
Bart Sanders, CEO
T: +31 40 851 64 31
E: info@stentit.com
FOR MEDIA
LifeSpring Life Sciences Communication | Amsterdam, The Netherlands
Leon Melens
T: +31 6 538 16 427
DISCLAIMER:
The Resorbable Fibrillated Scaffold is an investigational device,
not yet approved for sale in any jurisdiction.

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

